Novartis maintains a strong commitment to upholding and respecting human rights. In the Novartis Code of Ethics, we commit to “conduct our business in a manner that respects the rights and dignity of all people.” In 2020, our CEO became the first pharmaceutical CEO to sign the CEO Guide to Human Rights developed by the World Business Council for Sustainable Development.
In 2022, we updated our Human Rights Commitment Statement, endorsed by the executive-level Novartis ESG Committee. This is the latest update to our human rights statement, first adopted in 2003. Our Human Rights Commitment Statement establishes our foundational commitment to the International Bill of Human Rights, ILO Core Labor Conventions, and the UN Guiding Principles on Business and Human Rights (UNGPs).
It also explains the 4 human rights focus areas we’ve identified and outlines our methodology to embedding human rights throughout our business.
Our human rights priority areas
Governance & strategy
In 2021, the human rights program was integrated into the Ethics & Compliance team in the Ethics, Risk, and Compliance (ERC) function. The ERC function is led by the Chief Ethics, Risk, and Compliance Officer, who sits on the Executive Committee of Novartis (ECN).
Overall accountability for implementation of our human rights program sits with Novartis’ Chief Ethics, Risk, and Compliance Officer. The executive-level ESG Committee, chaired by the Novartis CEO, has endorsed our overall approach to managing human rights. A dedicated Human Rights team sits within the global ERC function and is responsible for the implementation of Novartis’ human rights strategy.
In 2021, we conducted a corporate-wide human rights risk assessment and prioritization workshop where we identified and prioritized our potential human rights risks and impacts, following guidance from the UNGPs. We used a human rights risk prioritization tool developed by BSR, an external NGO and advisor, to help guide our decision-making.
Our process was further informed by the findings from our 8 human rights country-level assessments conducted since 2017, which included engagement with approximately 200 internal employees, 40 third party management teams and employees, and 12 civil society organizations. We also considered findings from other human rights risk investigations and assessments conducted since 2018.
As part of our analysis, we also considered comments from Shift, a business and human rights NGO. We also consulted with BSR on the outcome of the prioritization process, and with an additional external human rights expert advisor, on the final draft of the Human Rights Commitment Statement. All these engagements resulted in material changes to our priority areas, and we are grateful for their collective guidance and support.
Our Human Rights Commitment Statement explains our 4 human rights focus areas and is based on four years of targeted human rights due diligence across the company. We refreshed our human rights strategy with a targeted, risk-based approach to managing human rights, and provide a framework to track our progress implementing the UNGPs.
How we deliver on our commitments
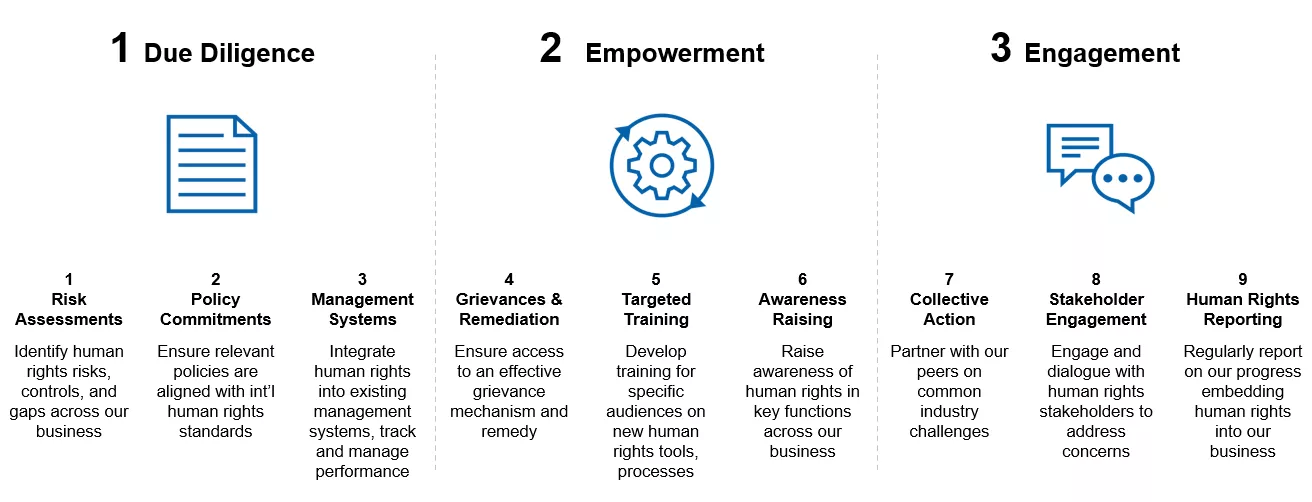
Ongoing human rights due diligence
Since 2017, we have conducted targeted human rights assessments across the business in our priority areas. These human rights assessments take a variety of forms:
- In Country-Assessments: We conduct in-country assessments to understand our potential human rights risks and impacts across our business operations in high-risk operating environments. Since 2017, we have conducted 8 in-person country human rights assessments. The program was placed on-hold during the pandemic due to travel restrictions. We are currently piloting a remote assessment approach in 4 markets. Countries are selected for an assessment based on a Human Rights Country Risk Assessment tool we developed, incorporating 14 publicly available human rights risk indicators, and the size of our operational footprint in the market. In 2019, we redesigned our human rights assessment program to include direct engagement with suppliers, communities, and civil society actors. For instance, our assessments in India, Singapore, Brazil, and China included supplier site visits as well as interviews with communities surrounding our or our suppliers’ operations, and relevant nongovernmental organizations.
- Business-Unit Assessments: We conduct targeted business-unit assessments to understand how human rights risk may arise in a particular part of the business, regardless of the geography. For example, we have completed assessments of our risk exposure in the procurement of raw materials, our main grievance mechanism (SpeakUp), procurement of human biosamples, the global policy framework for our clinical trials division, and other business unit areas.
- Rapid response "hot spot" assessments: We conduct rapid human rights assessments in response to emergency or "hot spot" issues that may arise where our business may potentially be exposed to human rights risk or impact. These have included, for example, assessments and responses to the Covid-19 health crisis and various geopolitical issues arising over the last several years.
- Business Development & Licensing (BD&L) Deal Review: In partnership with our third-party labor rights colleagues, we review potential labor and human rights risks of potential in-scope partners in merger, acquisition, licensing, or other corporate deal structures.
Due diligence scope & tools
We have developed a baseline human rights due diligence toolkit to support the types of assessments outlined above. The toolkit includes assessing the ILO Core labor rights (forced labor and human trafficking, child labor, freedom of association and the right to collective bargaining, equal remuneration, and non-discrimination) across all our operations.
Third party labor rights assessments & modern slavery
We assess all our in-scope third parties for labor rights. Corrective and Preventative Action Plans (CAPAs) have been put in place where issues were identified and are being monitored by Novartis risk experts. More details about these assessments can be found in the Novartis in Society Integrated Report 2023 (PDF 8.4 MB).
We continue to take steps to prevent modern slavery in our operations and supply chains. We publish an annual statement explaining how we address modern slavery risks or impacts and have developed an e-learning module on modern slavery.
For past modern slavery statements click here 2018 (PDF 0.3 MB), 2020 (PDF 2.2 MB), 2021 (PDF 0.4 MB), 2022 (PDF 0.4 MB).
In 2023 we published a statement in accordance with the Norwegian Transparency Act.
Stakeholders considered
The scope of our human rights due diligence considers all stakeholders and rightsholders who may be at risk of human rights impacts. These include our own employees, employees at third parties, patients, local community members, and groups that are generally considered to be at greater risk of vulnerability for harm (women, children, LGBTI people, people with disabilities, migrant workers, indigenous people).
As we expand our efforts on human rights, we plan to conduct broader and more frequent consultations with these groups. This will help us benchmark our efforts, measure our progress, and fulfill our ambition to become a leader in the healthcare sector for respecting and protecting the rights of people affected by our or our suppliers’ operations.
Risk identification & remediation
After conducting a human rights assessment, if an issue is identified, we assign a gap closure action plan or other risk mitigation approach to address all risks identified. We prioritize addressing mitigation measures that prevent the greatest harm to the greatest number of people, consistent with the UNGPs. Since December 2019, we have closed 33 of 78 mitigation measures identified across the company.
Own operations
Examples of risk mitigation and remediation measures taken within our own operations include:
- SpeakUp grievance mechanism
The Novartis SpeakUp Office, which from 2021 has been integrated into the Ethics, Risk & Compliance (ERC) function, enables employees and external parties to raise concerns about potential misconduct while being protected against retaliation.
In 2021, we completed a targeted human rights assessment of our SpeakUp grievance mechanism against the UNGPs. While we prohibited retaliation against anyone for raising a concern in our SpeakUp policies, during our assessment we identified the absence of a standalone Non-Retaliation Policy as a potential risk that may prevent people from raising concerns. To mitigate this risk, in 2022 we adopted a new standalone Non-Retaliation Policy to clarify and reinforce the message that retaliation against anyone for raising a SpeakUp case, or a grievance through any channel, will not be tolerated.
More details about the type of complaints and how they were handled can be found in the Novartis in Society Integrated Report 2023 (PDF 8.4 MB).
- Living wage in our operations
In 2000, Novartis was one of the first international companies to implement a commitment to pay a living wage to all its employees. Each year, Novartis Group companies review salaries for all associates and adjust salaries that fall below the living wage level. In 2021, we covered 97 countries, and we identified 120 cases across three countries where employee wages were below the agreed living wages. Based on the results, our local People & Organization teams made the relevant adjustments. More details about our living wage program can be found here.
- Diversity, equity and inclusion
We are working with our Diversity, equity and inclusion team to review policies affecting LGBTQI+ associates, with the objective to identify and close gaps from a human rights standpoint. More details about our Diversity, equity and inclusion work are available here.
- Disability inclusion
We are supporting the development of the first Novartis global strategy on disability inclusion to ensure a human rights-based approach guides the design and implementation of this strategy (i.e., engaging with rights-holders throughout the process, viewing risk from a rights-holder perspective, addressing all forms of disability).
Third parties & supply chain
Examples of risk mitigation and remediation measures taken with our third parties and supply chain:
- Third Party Code Update
In January 2023, we have updated our Third Party Code to further specify human rights due diligence, environmental compliance, and environmental sustainability expectations from third parties. Our Third Party Code is consistent with the Pharmaceutical Supply Chain Initiative principles for responsible supply chain management and part of vast majority of our contracts with third parties.
- Modern slavery risk mitigation
Please see our latest annual Modern Slavery Statement for more details on how we work on risk remediation with Tier 1 suppliers, capacity building pilot projects, and collective action projects.
Training and capacity building
We continue to develop and roll out training on human rights for associates. We work with associates who are confronted with our most important issues to develop the content of our training modules. Highlights include:
- Human Rights Ambassador Network: We established this network within the Ethics, Risk, and Compliance (ERC) function to build capacity among associates across markets on issue identification and analysis from a human rights perspective.
- Labor rights: We delivered a workshop for our global Third-Party Labor Rights managers to explain the four core ILO labor rights and how they apply to our business.
- Modern slavery: We developed an online training for procurement, ERC, and associates in the UK and Australia (where modern slavery reporting is legally required).
External engagement
Novartis is engaged in several collaborative efforts to advance human rights in the healthcare sector and across industries.
Pharmaceutical Supply Chain Initiative (PSCI)
Novartis has held several leadership positions in the PSCI, including Co-chair of the Human Rights & Labor (HRL) subcommittee. Our Chief Ethics, Risk and Compliance Officer serves on the PSCI External Advisory Committee.
Novartis has initiated several human rights projects and activities in the PSCI, including a project in the Partnerships Committee to mobilize collective PSCI action to contribute to the State of Telangana’s Musi River Revitalization Initiative in Hyderabad, India. We have also led collective action projects to address the allegations of forced labor in the carnauba wax supply chain and developed several training programs on human rights.
BSR working group on human rights
Novartis has participated in BSR’s cross-industry Human Rights Working Group (HRWG) since 2018. The HRWG provides a platform for sharing cross-industry best practices and for identifying emerging business and human rights issues.
Codes, policies and resources
We are embedding human rights into all relevant Novartis policies and procedures.