With the advancement of T-ChargeTM—a new Novartis-developed platform for investigational pipeline CAR-T cell therapies—we are underscoring our commitment to pioneering cutting-edge innovation in transformative cell therapies.
Going beyond incremental advances
Bringing potentially curative cell therapies to more patients, and providing people living with cancers the possibility of living cancer free, has continuously been our vision.
T-Charge is a next generation platform through which we aim to revolutionize CAR-T cell therapy, potentially leading to new products that may offer patients a higher likelihood of better and more durable responses with the ultimate potential for a cure1.
Using key learnings from other CAR-T therapies, we are hopeful that our next-generation CAR-T treatments will be delivered to patients faster and benefit a broader patient population than ever before.
Jennifer Brogdon, Executive Director, Head of Cell Therapy Research, Exploratory Immuno-Oncology at Novartis
How does T-Charge work—and how could patients benefit?
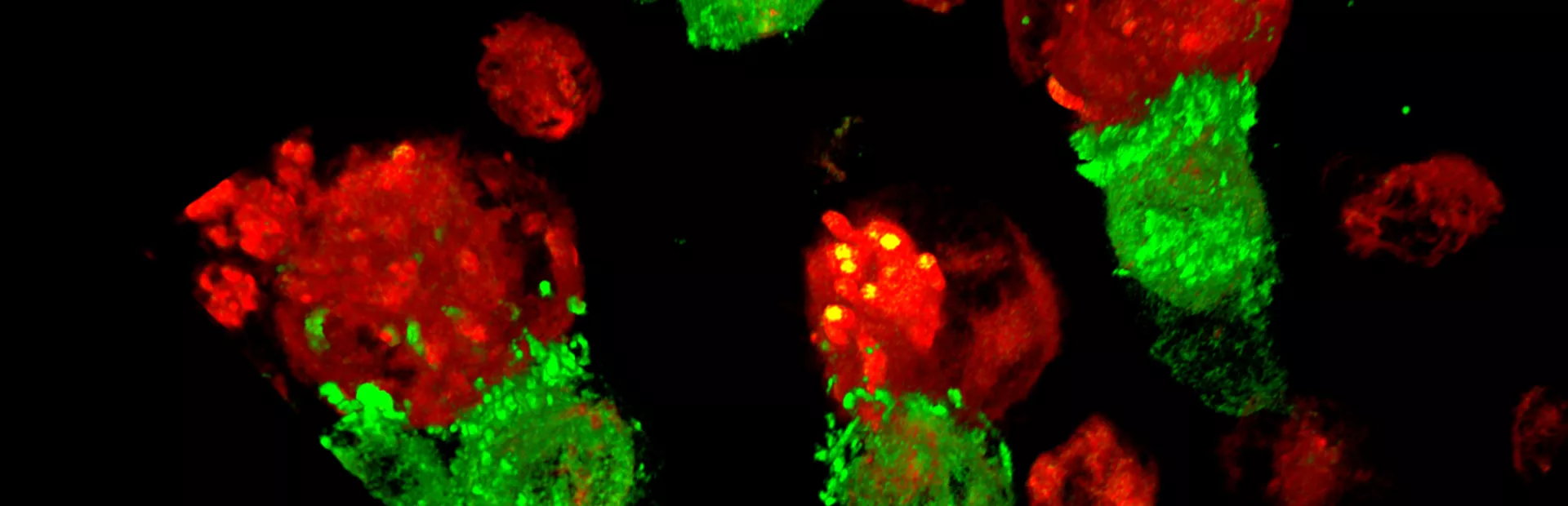
With T-Charge, CAR-T cell expansion occurs primarily within the patient’s body (in-vivo), eliminating the need for an extended culture time outside of the body (ex-vivo).
T-Charge preserves T cell stemness (the ability to self-renew and mature), which results in a product containing greater proliferative potential and fewer exhausted T cells. These unique characteristics of the T-Charge platform may lead to improved patient outcomes with reduced risk of severe adverse events1.
T-Charge is uniquely exciting because we’ve developed an improved way to handle these cells before they go back to the patient: Enabling the patient to be their own ‘bioreactor,’ so the cells can do the expansion and act naturally within their bodies.
Boris Engels, Associate Director at Novartis Institutes for BioMedical Research (NIBR)
Revolutionizing the CAR-T cell therapy experience
The Novartis T-Charge platform is being studied in first-in-human clinical trials across various indications, and it aims to impact the process and experience in numerous ways1,2:
- Rapid cell manufacturing process time compared with traditional CAR-T
- Reliable delivery through simplified processes and streamlined quality control
- Flexibility in setting of care (allowing for outpatient and community setting treatment, as well as, inpatient treatment)
As a leader in cell & gene therapy, we are committed to continuously evolving our CAR-T cell therapy offerings to further support more patients with high unmet needs.