By spying on cancer cells, Novartis researchers have a made a preliminary discovery that could potentially shape how targeted therapies are combined to treat patients. Currently doctors use the genomic profile of a patient’s tumor to select drugs that will hit the right target and shrink the cancer. Targeted therapy has prolonged many lives, but most cancers come back, and these drug-resistant tumors are very difficult to treat.
This disappointing outcome is practically a given, according to data published online April 13 in Nature Medicine. The paper describes the researchers’ “spy data,” which suggests that many if not most tumors contain a few cells, covert cells, if you will, that already have the molecular tools to resist therapy before it even starts. These covert cells are so rare that they are nearly impossible to detect in tumors prior to treatment with current diagnostic tools. As a result, some researchers concluded that they did not exist at all.
“It was unclear whether drug resistance preexists or if some cells randomly develop resistance mutations during treatment,” says first author Hyo-eun (Carrie) Bhang, an investigator in the Novartis Institutes for BioMedical Research (NIBR) oncology unit.
This new evidence confirming the existence of rare drug-resistant cells in tumors upends current thinking about targeted therapy.
“Our finding that rare resistant cells are already present prior to therapy may explain the short duration of response to most targeted cancer agents,” says lead detective Frank Stegmeier, a director in NIBR oncology. “If we can devise strategies to target these pre-existing resistance cells, we may have a chance of eradicating some patients’ cancers.”
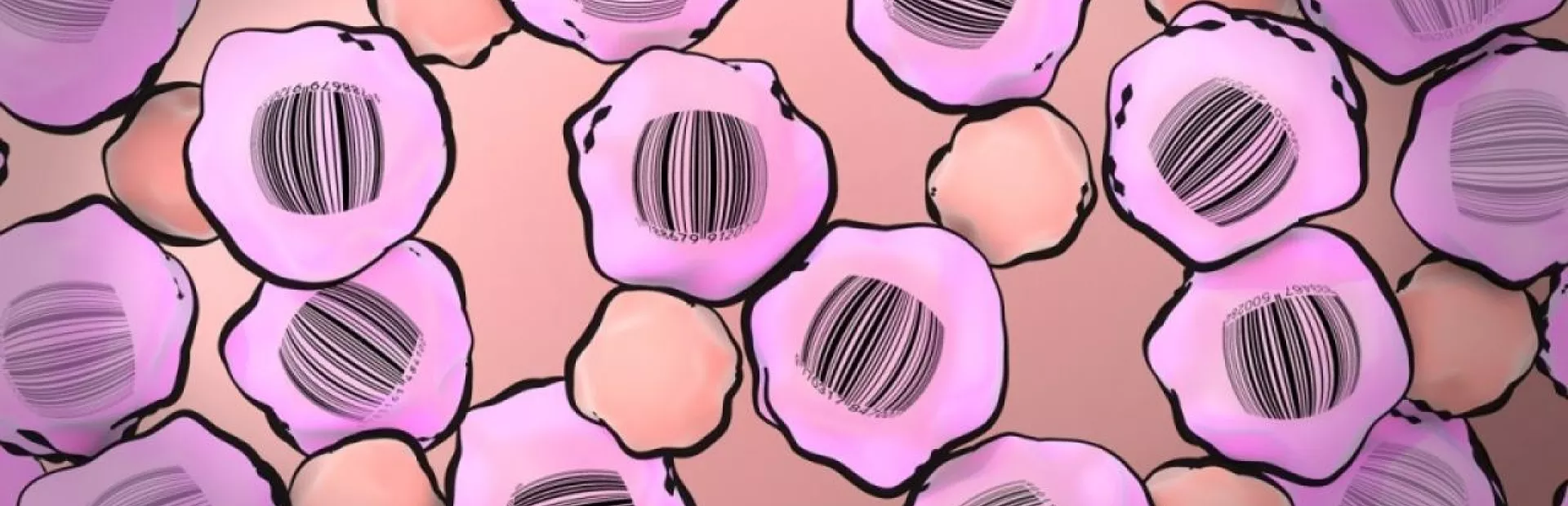
To understand resistance and possibly find a way to beat it, Bhang and Stegmeier developed ClonTracer, a surveillance system that applies labels akin to molecular barcodes inside tumor cells and is capable of uniquely labeling millions of cells. As the cells divide, daughter cells retain the barcodes of their ancestors; so all offspring of a given cell will share the same label.
ClonTracer made it possible for Stegmeier and Bhang to see which of the offspring of cells in a tumor sample survive drug therapy. They used this information to determine whether the survivors were descendants of cells that had drug resistance mutations all along but were too rare to detect using current next generation sequencing technology, or if they developed resistance as the tumor cells mutated and evolved over time.
For example, Bhang applied one million barcodes to cultured cancer cells from a non-small cell lung cancer model known to be sensitive to a certain drug but also to develop resistance to it. She created eight copies of the labeled sample, making sure that each of the copies contained offspring representing all of the million barcodes. Bhang then treated all eight copies with the same drug, essentially performing the same experiment eight times in parallel, and waited to see which cells survived.
The results of this experiment were very striking. In all eight samples, of the one million unique barcodes represented, only 400 to 500 survived. If these cells developed resistance during treatment through random mutations, the barcodes of the surviving cells would also be expected to be random. That is, the survivors would have emerged from different ancestor cells in each experiment.
Bhang found the opposite. Cells with the same barcodes survived across the eight samples. Nearly half were the same across all eight. Over and over, offspring of the same ancestors resisted the drug. “The chances of this happening randomly is astronomical,” says Stegmeier. “The evidence was overwhelming that these resistant cells must preexist in the cancers.”
Mathematical modeling, based on the team’s quantitative spy data and done in collaboration with Dana-Farber Cancer Institute biostatistician Franziska Michor, strongly supported this finding.
Bhang repeated the experiment in a cell culture model of chronic myeloid leukemia. This time, she independently tested two drugs that target the same cancer driver, but in different ways. She found that both drugs killed most of the cells – 99.9995 percent of the cells in one case – but the barcodes of the cells that repeatedly survived treatment with one drug did not match those of the other, indicating that the preexisting resistance to one drug was different than that of the other drug.
This observation led to an idea. “If we try an up-front combination of these two drugs, maybe we can kill off both of these resistant populations and prevent resistance,” says Bhang.
Until now, drug developers have mostly been working under the assumption that resistance emerges during treatment, so they have adopted a strategy for combined therapy that focuses on killing more cells faster, reducing the likelihood that new resistance mutations will emerge. Unfortunately, killing more cells faster is futile if even a single resistant covert cell already exists. It will survive, thrive, and feed the growth of a tumor with even more resistance mechanisms. In other words, says Stegmeier, “tumor evolution will win.”
By pairing drugs that are evaded by different mechanisms, one drug kills the rare covert cells resistant to the other, and vice versa. The researchers plan to continue use of the ClonTracer technology to pinpoint complementary drug combinations.
“This gives us a very precise way to measure the relative effect of combination drugs and specifically how overlapped they might be,” says William Sellers, Global Head, Oncology, at NIBR. “In turn, this could help determine the curative potential for distinct combinations.”
Novartis researchers have already begun testing just such a combination in mouse models. So far, the results are showing some promise that a clinical trial might be in the works to test the pairing in humans at some point.
Artist Mark Mazaitis’s interpretive illustration of cancer cell “barcoding.” A description of the technology, ClonTracer, appears above.